The following table summarizes the influence each of the reducing systems discussed above has on the different classes of carboxylic acid derivatives. Note that LAH is the strongest reducing agent listed, and it reduces all the substrates.
The following table summarizes the influence each of the reducing systems discussed above has on the different classes of carboxylic acid derivatives. Note that LAH is the strongest reducing agent listed, and it reduces all the substrates.
 In a similar sense, acyl chlorides are the most reactive substrate. They are reduced by all the reagents, but only a few of these provide synthetically useful transformations.
In a similar sense, acyl chlorides are the most reactive substrate. They are reduced by all the reagents, but only a few of these provide synthetically useful transformations.
 4. Other Reactions
4. Other Reactions
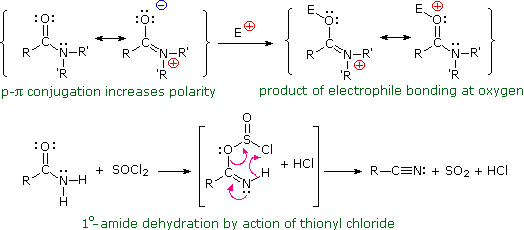
 Amides are very polar, thanks to the n-π conjugation of the nitrogen non-bonded electron pair with the carbonyl group. This delocalization substantially reduces the basicity of these compounds (pKa ca. –1) compared with amines (pKa ca. 11).
Amides are very polar, thanks to the n-π conjugation of the nitrogen non-bonded electron pair with the carbonyl group. This delocalization substantially reduces the basicity of these compounds (pKa ca. –1) compared with amines (pKa ca. 11).
 When electrophiles bond to an amide, they do so at the oxygen atom in preference to the nitrogen. As shown below, the oxygen-bonded conjugate acid is stabilized by resonance charge delocalization; whereas, the nitrogen-bonded analog is not.
When electrophiles bond to an amide, they do so at the oxygen atom in preference to the nitrogen. As shown below, the oxygen-bonded conjugate acid is stabilized by resonance charge delocalization; whereas, the nitrogen-bonded analog is not.
 One practical application of this behavior lies in the dehydration of 1º-amides to nitriles by treatment with thionyl chloride. This reaction is also illustrated in the following diagram. Other dehydrating agents such as P2O5 effect the same transformation.
One practical application of this behavior lies in the dehydration of 1º-amides to nitriles by treatment with thionyl chloride. This reaction is also illustrated in the following diagram. Other dehydrating agents such as P2O5 effect the same transformation.